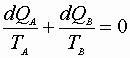
Open System – 1st Low
![]()
![]()
![]()
![]()

![]()
![]()
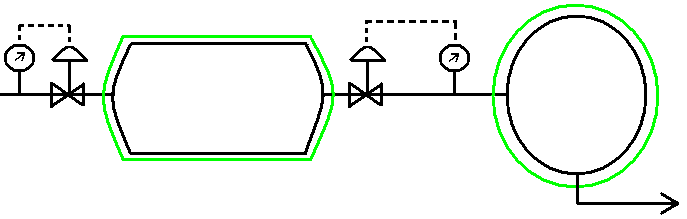
T = 366 K Pinitial = 1 bar
P = 2 bar Tinitial = 311 K
Gas (air): Cp = 29.3 J/mol K
Non-steady State analysis:
![]()
![]()
![]()
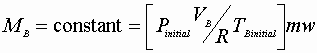
The 1st Law reduces to:
![]()
or
![]()
Integration from a® b & c® d:
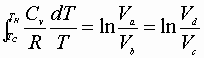
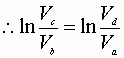
Dividing equation A by B yields:
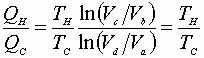
Now we can express:
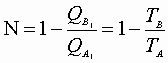
For this reversible process: C may be written:
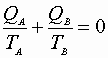 or
or